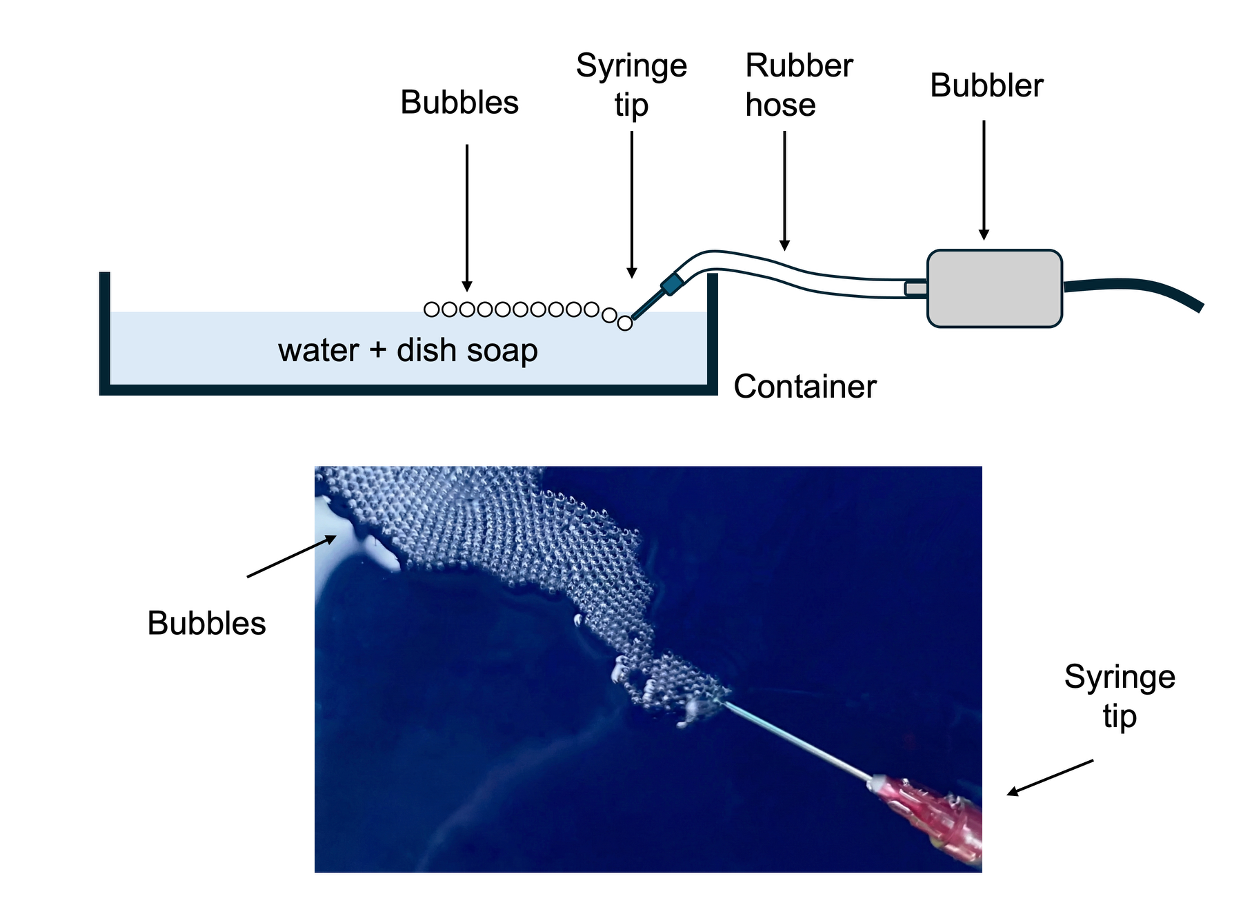
Creation of 2D bubble crystals
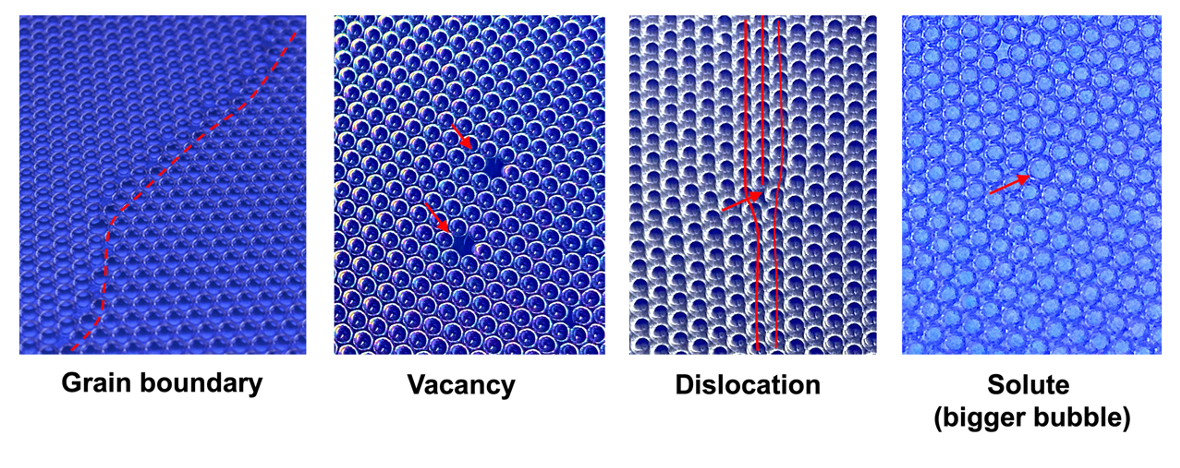
A crystal consists of atoms arranged in a periodic, repeating pattern. Interestingly, you can create a two-dimensional analogue of a crystal using bubbles floating on water. This bubble-crystal model not only makes it possible to visualize how atoms organize into a lattice, but also lets you deliberately introduce various imperfections such as vacancies, solute atoms, dislocations, and grain boundaries. Even more exciting, you can perform simple mechanical tests on this model. You can observe how plastic deformation occurs through dislocation motion and how fracture initiates along grain boundaries – phenomena that are often difficult to see directly in real materials.
All of these demonstrations are surprisingly accessible if you have the following items.
- Water
- Dish soap
- Container
- Fish tank bubbler
- Rubber hose
- Syringe needle
The key component in this experiment is the fish-tank bubbler. To form a proper bubble crystal, all bubbles must be the same size (typically a few millimeters in diameter). Because a fish-tank bubbler supplies a steady, consistent airflow, it allows you to generate bubbles of uniform size continuously.
First, fill a container with water and mix in dish soap. The required amount of soap varies by brand, so simply add more until the bubbles form easily and do not pop too quickly.
Second, assemble the bubbler by connecting the bubbler, rubber hose, and syringe needle. Smaller bubbles are generally better for forming a crystal, so a syringe needle with a small opening works best. If the needle produces bubbles that are too large, you can gently crimp the needle tip with a tool to reduce its diameter. If the bubbler’s airflow is too strong, you can also create a few tiny holes in the rubber hose to let some air escape; this will reduce the flow rate at the syringe needle. (Note: The syringe needle is optional. If you don’t have one, you can simply create bubbles directly from the rubber hose. For example, you can seal the end of the hose and make a tiny hole on its side; bubbles will then form at that opening.)
Third, turn on the bubbler and place the syringe needle into the soapy water. You should quickly see uniformly sized bubbles forming. If the bubbles rise too quickly and begin to pile up, gently blow them aside with your breath (or use a hair dryer on a low setting or portable fan). Soon, you’ll observe the bubbles attaching to one another and arranging themselves into a periodic pattern. This attraction occurs because of surface tension, which pulls bubbles together—much like how atoms bond due to chemical forces.
Microstructures in Bubble Crystals
Grain boundaries and impurities

Bubble nanowire tension 1
Bubble nanowire tension 2
Are you able to see the motion of dislocations?
Dislocation plasticity 1
Are you able to see the motion of dislocations?
Dislocation plasticity 2 and grain boundary fracture
Are you able to see the motion of dislocations?